The UK’s decision to lengthen the gap between first and second doses of the Pfizer and AstraZeneca vaccines has been criticised by some. But what if we had a vaccine which only needed one dose, and had been tested on that basis? A vaccination programme could progress far more quickly and without the complication of having to ask people to come back to a clinic a second time. In particular, it would simplify the distribution of vaccines in countries with less-well developed public health systems.
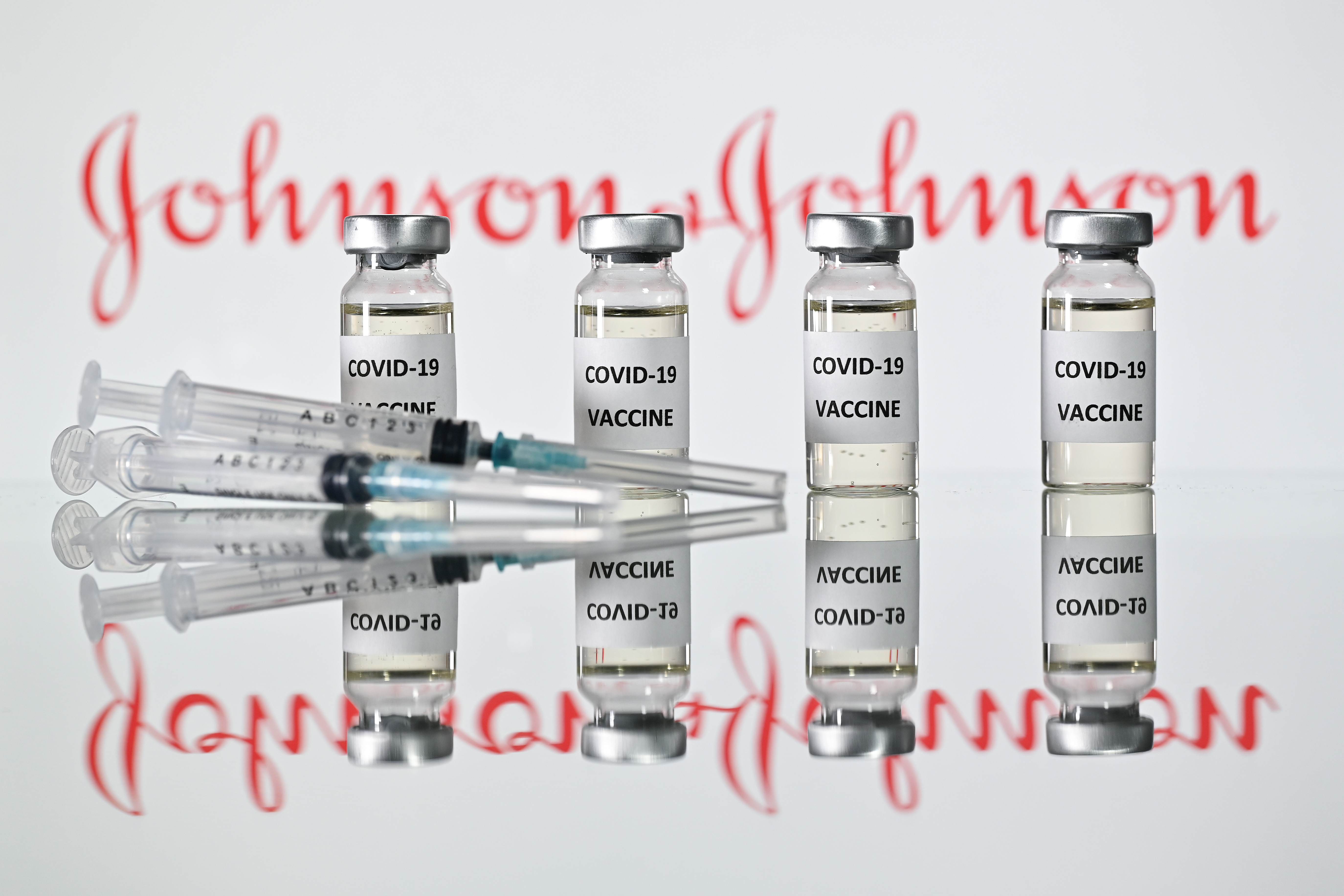
That is the promise of the latest vaccine candidate for which results have been reported: the Johnson & Johnson vaccine, which is based on an existing Ebola jab. However the results, published in the New England Journal of Medicine, cover only phases one and two of the trial — which measure the response of the immune system to the vaccine and other safety aspects.
Get Britain's best politics newsletters
Register to get The Spectator's insight and opinion straight to your inbox. You can then read two free articles each week.
Already a subscriber? Log in








Comments
Join the debate for just $5 for 3 months
Be part of the conversation with other Spectator readers by getting your first three months for $5.
UNLOCK ACCESS Just $5 for 3 monthsAlready a subscriber? Log in