Over the past few weeks the government’s scientific advisers have indicated that the only real way out of the coronavirus crisis is a vaccine – until then a high degree of social distancing will have to remain. Given that no-one expects a vaccine to be ready for deployment for another year at the very earliest, this would have very serious implications for the economy, and for society at large.
But there is another possible route out of social distancing – not so good, but possibly available a lot more quickly. This is for an effective therapeutic drug to emerge. It would not stop anyone contracting the virus but could improve the prospects for those who do catch it. If the death rate and the reliance on artificial ventilation could be greatly reduced would there be any need for social distancing?
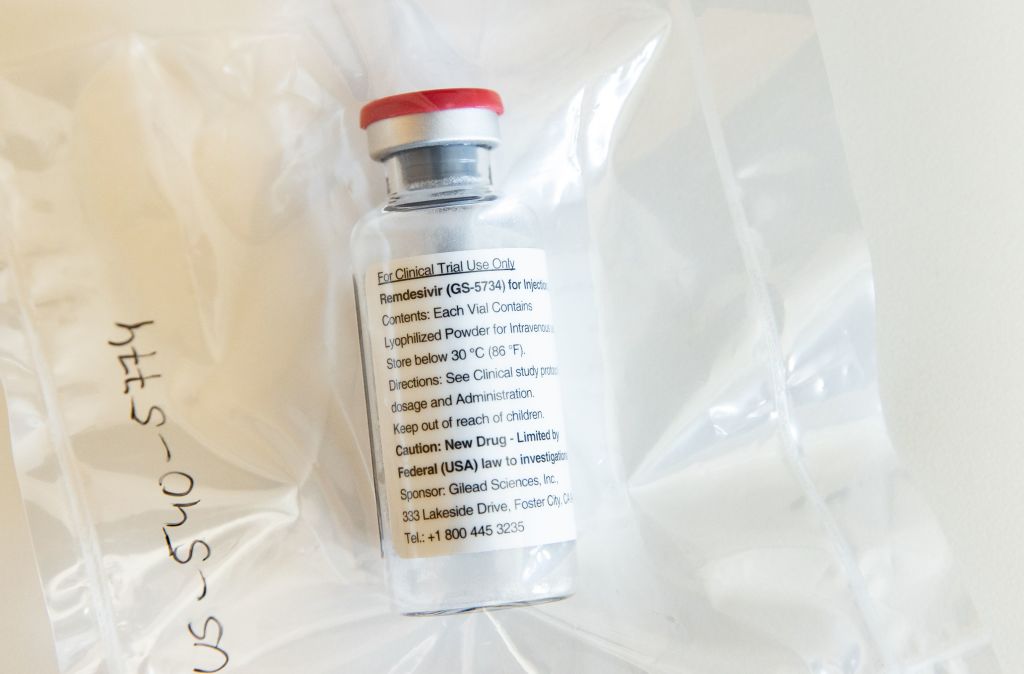
This afternoon comes a potentially important breakthrough in the search for a safe and effective anti-viral drug against Covid-19. Californian drugs company Gilead Sciences has put out two statements regarding trials into Remdesivir. The first was the results of its own study into patients who have been hospitalised with a severe manifestation of the disease and were given five and 10 day courses of the drug. Of patients administered with the drug more than half were able to be discharged from hospital 14 days later. Seven per cent had died by day 14. The company plans to publish its full results over the next few weeks.
The other statement simply stated that the company is aware of ‘positive’ results from a trial of the drugs by the National Institute of Allergy and Infectious Diseases. According to the statement, the trial has reached its ‘primary endpoint’, suggesting that it could be heading for approval by the Food and Drug Agency. A further statement is expected later.
This is significant because it would appear to contradict reports from last Friday suggesting that results of the trials had been disappointing. This afternoon’s statement has certainly excited investors – trading in Gilead’s shares had to be suspended for a while as the news was digested. The wider stock market rose strongly after the news.
It is only a therapeutic drug, not a vaccine, and it could yet fail. Nevertheless, it is a reminder that the climate surrounding Covid-19 could change very quickly with the emergence and approval of effective drugs. The gloomy prospect of having to suppress freedom and economic life for a whole year or more while we wait for a vaccine may be lifted early.
This article is free to read
To unlock more articles, subscribe to get 3 months of unlimited access for just $5







Comments
Join the debate for just $5 for 3 months
Be part of the conversation with other Spectator readers by getting your first three months for $5.
UNLOCK ACCESS Just $5 for 3 monthsAlready a subscriber? Log in